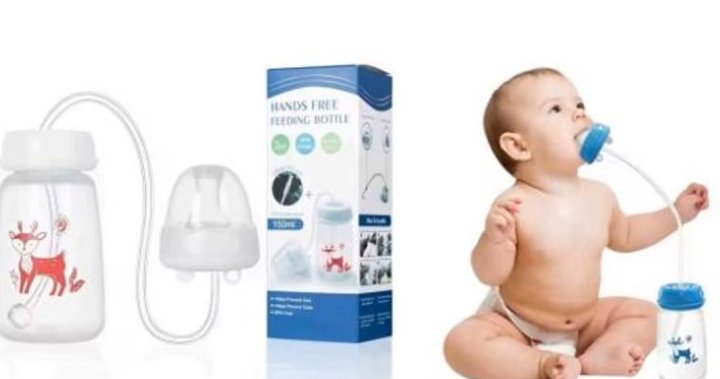
Health Canada has issued an urgent advisory strongly urging parents and caregivers to immediately discontinue the use of infant self-feeding devices purchased from AliExpress.ca. These devices, prohibited in Canada, present significant choking and suffocation risks to infants, thereby violating the Canada Consumer Product Safety Act. The agency underscores the gravity of these risks, emphasizing the potential for severe health consequences, including illness and even death, due to aspiration of feeding liquids. Consumers are instructed to not only stop using these products but also to dispose of them safely.
The inherent danger of these self-feeding devices stems from their design, which allows infants to hold and control their bottles before they have developed the necessary muscular coordination and control. This premature self-feeding can lead to difficulties in regulating intake, potentially causing infants to ingest more liquid than they can safely handle. Furthermore, the design of these devices often hinders the infant’s ability to stop feeding when necessary, exacerbating the risk of choking or aspiration. Healthy infants often regurgitate small amounts of liquid during feeding; these devices increase the likelihood of aspirating such regurgitated fluid, particularly without close adult supervision.
Health Canada emphasizes the importance of constant monitoring during infant feeding. Caregivers should be vigilant for any signs of difficulty or distress, such as excessive fluid intake, unusual feeding behaviors, or regurgitation. Immediate intervention is crucial should any concerning signs arise. This advisory highlights the need for caregivers to actively supervise feeding sessions and ensure the infant’s safety. The agency’s proactive approach aims to prevent potential harm by alerting parents and caregivers to the hazards associated with these banned devices.
The specific products included in this advisory are various types of self-feeding bottles, often marketed with features like long straws, hands-free designs, rotating cups, and leak-proof claims. These products are commonly sold under various names and branding, with listed capacities ranging from 300 to 330 milliliters. While the specific product names listed in the advisory are helpful, parents and caregivers should be wary of any product that allows an infant to self-feed without direct supervision and the ability to easily control the flow of liquid. The common feature across these banned products is the mechanism that allows the infant to independently manage the bottle, thereby creating the inherent risk of choking or suffocation.
Following the identification of these hazardous products, Health Canada has taken swift action. The agency has successfully removed the listed self-feeding devices from AliExpress.ca, effectively halting their online sale in Canada. Furthermore, Health Canada has contacted the foreign third-party sellers involved, communicating the Canadian ban and the associated safety concerns. This proactive engagement aims to prevent further distribution of these banned devices. While AliExpress.ca reported selling 60 units of these products in Canada, no incidents or injuries related to their use have been reported to Health Canada as of the advisory’s release.
This advisory underscores Health Canada’s commitment to consumer safety, especially concerning vulnerable populations like infants. The agency’s prompt action in identifying, banning, and removing these hazardous products from the Canadian market demonstrates a proactive approach to preventing harm. By actively engaging with both consumers and sellers, Health Canada aims to minimize the risk posed by these non-compliant products. The advisory’s emphasis on immediate cessation of use and proper disposal of these devices highlights the serious nature of the associated risks.